Cardinal Health MarketSM
The legacy Cardinal.com Medical Ordering site has been replaced with Cardinal Health MarketSM, a new product experience designed with you in mind.
DUBLIN, OHIO – August 19, 2021 — On August 4, 2021, Cardinal Health (NYSE: CAH) initiated a nationwide recall of approximately 267 million MonojectTM Flush Prefilled Saline Syringes (0.9% Sodium Chloride). The products have been found to reintroduce air into the syringe after the air has been expelled. This could result in injection of air into blood vessels and create the potential for air embolism, which can cause serious adverse health outcomes or death.
Customers who have affected product(s) should immediately review their inventory and quarantine and return all affected product. The recall applies to all lots of the products manufactured from July 2019 to June 2021 distributed between July 2019 and July 2021.
The following SKUs have been recalled:
|
Name of Product |
SKUs |
|
12mL Syringe, 10 mL Saline Fill |
8881570121 |
|
12mL Syringe, 3 mL Saline Fill |
8881570123 |
|
12mL Syringe, 5 mL Saline Fill |
8881570125 |
Our Monoject™ 3mL syringe, 2.5mL Saline Fill is not affected by this recall. This item, SKU 8881570300, will be available for sale until inventory is depleted.
MonojectTM Flush Prefilled Saline Syringes can be identified by the barrel of the syringe, the case and the box as shown in the pictures below:
| Barrel Print | Box Print | Case Print |
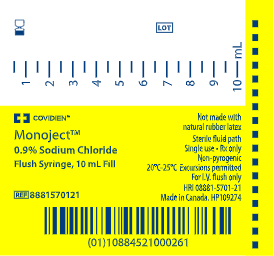 |
 |
 |
The company has received 37 reports of the plunger pulling back. No injuries have been reported to date.Cardinal Health voluntarily recalled MonojectTM Flush Prefilled Saline Syringes after learning that there is potential for the syringe’s plunger to draw back and reintroduce air back into the syringe. Cardinal Health has notified the U.S. Food and Drug Administration of this action.
Cardinal Health notified its distributors and customers by overnight delivery of a notification packet on August 5, 2021 and is arranging for return of all recalled products.
Cardinal Health distributes this product nationwide.
Customers with questions may contact the market action team via telephone at 800-292- 9332 between the hours of 8 a.m. – 5 p.m. EST. Customers may also contact the team via email at GMB-FieldCorrectiveAction@cardinalhealth.com.
Adverse reactions or quality problems experienced with the use of these products may be reported to the FDA's MedWatch Adverse Event Reporting program online, by mail or by fax:
Given the limited number of alternative prefilled saline syringe options, combined with ongoing supply, transportation and labor challenges, we anticipate that this recall may contribute to a market disruption of prefilled saline syringes. We are actively working to address the disruption and support customers’ needs with locating alternative supply and we suggest that clinicians work with their facility to determine alternative fill methods.
For more information and the latest updates, please visit cardinalhealth.com/MonojectFlushPrefilledSalineSyringesIssue.
About Cardinal Health
Cardinal Health is a distributor of pharmaceuticals, a global manufacturer and distributor of medical and laboratory products, and a provider of performance and data solutions for healthcare facilities. With 50 years in business, operations in more than 35 countries and approximately 44,000 employees globally, Cardinal Health is essential to care. Information about Cardinal Health is available at cardinalhealth.com.
Contacts
Media: Andrew Stern, andrew.stern@cardinalhealth.com and (614) 339-4678