Cardinal Health MarketSM
The legacy Cardinal.com Medical Ordering site has been replaced with Cardinal Health MarketSM, a new product experience designed with you in mind.
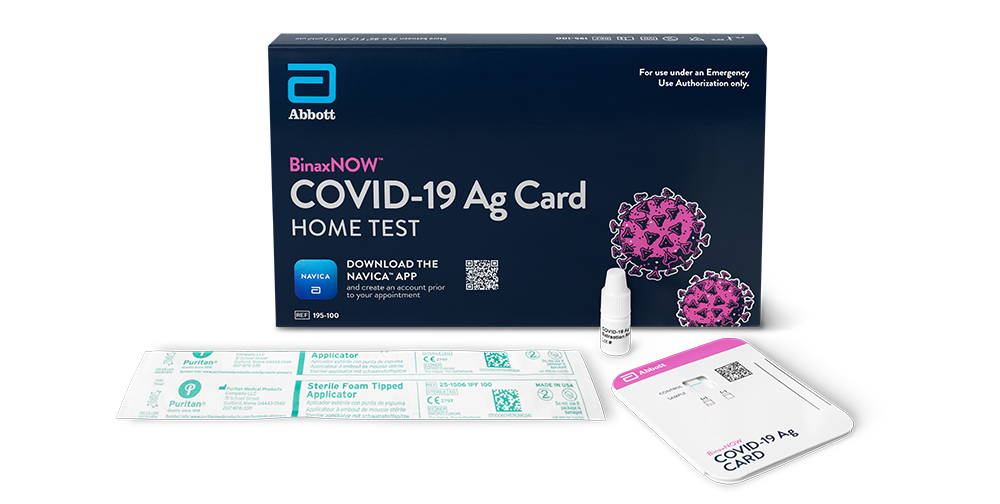
MIAMI — March 8, 2021 — eMed™, a digital health company democratizing healthcare through digital point-of-care solutions, and Cardinal Health (NYSE: CAH), a healthcare distributor, global manufacturer and solutions provider, today announced a collaboration to distribute millions of at-home COVID-19 rapid tests to pharmacies across the continental United States, Puerto Rico and U.S. Virgin Islands.
“There is a significant need for affordable, easily accessible coronavirus testing in this country now and in the future,” said Dr. Patrice Harris, eMed CEO. “This collaboration with Cardinal Health puts us closer to consumers, providing them with pick-up options at convenient pharmacy locations they frequent, know and trust.”
eMed offers a unique digital health platform to virtually guide individuals through the testing process by an eMed Certified Guide. The tests require no additional equipment, are authenticated and deliver results within minutes.
Under the agreement with Cardinal Health, the eMed tests will be distributed to pharmacies nationwide and be available for consumer pickup. Users will complete a questionnaire form at eMed.com, then present the completed form at a participating pharmacy to be evaluated by a pharmacist. Following their consultation with a pharmacist, they receive a test for at-home administration if eligible.
The testing process is virtually guided remotely by an eMed Certified Guide who will authenticate the test and result, automatically reporting to public health departments for tracking and tracing, thus benefitting broader public health.
“This collaboration will enable all pharmacies nationwide to offer convenient, at-home tests to their patients, and help protect against the further spread of COVID-19 in their communities,” said Victor Crawford, Pharmaceutical Segment CEO for Cardinal Health. “We are pleased to work together with eMed and use our preeminent distribution network to ensure these tests can be easily accessed by all pharmacies in the continental United States, Puerto Rico and U.S. Virgin Islands to fight the pandemic.”
Between the collaboration with Cardinal Health, individual sales, and partnerships with state governments, businesses and organizations, eMed plans to distribute and administer millions of tests throughout the country.
About eMed
eMed (www.eMed.com) is a digital health company democratizing healthcare with a digital point-of-care platform that provides fast, easy and affordable at-home health care testing, supervised and guided online by eMed Certified Guides. We embrace quantitative medicine to deliver prescribed tests and treatments directly to patients, driving better and more cost-effective health outcomes.
About Cardinal Health
Cardinal Health is a distributor of pharmaceuticals, a global manufacturer and distributor of medical and laboratory products, and a provider of performance and data solutions for healthcare facilities. With 50 years in business, operations in more than 40 countries and approximately 48,000 employees globally, Cardinal Health is essential to care. Information about Cardinal Health is available at cardinalhealth.com.
For product inquiries or to learn more, email us: BinaxNOW-Inquiries@cardinalhealth.com
Contacts
Media: Victor Scott, victor.scott01@cardinalhealth.com and (614) 783-2408
Investors: Kevin Moran, kevin.moran@cardinalhealth.com and (614) 757-7942